Background: Non-Hodgkin lymphoma (NHL) is one of the most common hematological malignancies in the world. Despite advances in treatment options, such as chimeric antigen receptor T cells (CAR-T) or bispecific antibodies, many patients still recur after complete remissions (CR) or do not achieve CR. Since B cell malignancies arise from clonal expansion, the B cell receptor (BCR) expressed on the cell surface, idiotype, has been studied as a tumor-specific antigen. Several trials have evaluated idiotype vaccines in clinical setting, but failed to demonstrate efficacy. Limitations have arisen from the uncertainty of BCR sequence confirmation, long turnaround time in manufacturing idiotype antigen, and the absence of strong immune modulators. Here, we utilized targeted next generation sequencing (NGS) for BCR, cell-free protein synthesis (CFPS) system for idiotype antigen manufacturing, and a novel liposomal TLR7/8 agonist-based adjuvant to optimize idiotype vaccine.
Methods: To generate the idiotype antigens as single-chain variable fragment (scFv) from A20 lymphoma cell line and biopsy samples from NHL patients, variable regions of BCR heavy and light chain were sequenced by targeted NGS. Total RNA was isolated from A20 cells and tumor tissues from patients, and reverse transcription-polymerase chain reaction (RT-PCR) was performed using constant region-specific primers, resulting in the 1 st strand cDNA. Subsequently, the 2 nd strand cDNA was synthesized using V-gene specific primers and sequenced using an Illumina MiSeq sequencer. We applied targeted NGS to patients' tumor tissues from Asan Medical Center and Seoul National University Hospital to understand whether this method works in human tissues as well.
The identified sequence was cloned into a linear DNA template and expressed by CFPS system. We optimized the CFPS system and purification process of idiotype antigen scFv from A20 cell line and human tissues. The liposomal adjuvant ProLNG-001 (TLR7/8 agonist 140 μg) and ProLNG-002 (TLR7/8 agonist 70 μg + TLR4 agonist 5 ug) were synthesized by microfluidics method. Idiotype antigen A20-scFv 50 μg was given alone or mixed with ProLNG-001 or -002.
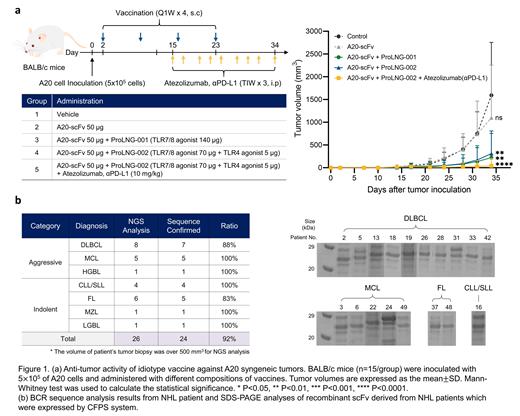
To investigate the efficacy of the idiotype vaccine, BALB/c mice were subcutaneously injected with 5×10 5 of A20 cells. Mice were subcutaneously vaccinated 4 times at 1-week intervals starting from day 2 after tumor inoculation. Atezolizumab 10 mg/kg was administered intraperitoneally 3 times a week for 3 weeks in the idiotype vaccine + immune checkpoint inhibitor combination group (Figure 1a).
Results: Targeted NGS has confirmed BCR sequences from A20 cell line and dominant malignant B cell clone from patients' tumor with a success rate of over 90% when sufficient volume (more than 500 mm 3) of biopsy sample was provided for RNA extraction. Also, we were able to express the idiotype scFv antigens determined from 18 NHL patients' tumor samples using CFPS system with a 100% success rate (Figure 1b).
The untreated mice (Group 1) showed a tumor growth pattern similar to that of Group 2 (vaccination with antigen only). Vaccination with idiotype antigen mixed with liposomal adjuvants, ProLNG-001 (Group 3) and ProLNG-002 (Group 4), significantly inhibited tumor growth. The combination of anti-PD-L1 antibody and idiotype antigen mixed with ProLNG-002 (Group 5) generated even better overall anti-tumor responses with 14 out of 15 mice showing no residual tumor at day 34 after tumor inoculation.
Conclusion: Taken together, the idiotype vaccine strategy using targeted NGS for BCR, CFPS system for idiotype antigen manufacturing, and TLR7/8-agonist-based liposomal adjuvants showed highly effective results in the preclinical syngeneic A20 tumor model. Furthermore, the usage of immune checkpoint inhibitors combined with idiotype vaccine in B cell lymphoma may exert synergistic effects. The CFPS system can be applied to swift, robust and personalized manufacturing of idiotype antigen for individuals with different idiotype sequence in a GMP setting, which was not possible in prior studies. These results support further development of the idiotypic vaccine as a treatment option for non-Hodgkin's B cell lymphomas.
Disclosures
Moon:Progeneer Incorporation: Current Employment. Chun:Progeneer Incorporation: Ended employment in the past 24 months. Kim:Progeneer Incorporation: Current Employment. Seo:Samsung Biologics: Current Employment. Kim:Progeneer Incorporation: Current Employment, Current equity holder in private company. Yoon:Pharos iBio: Consultancy; Novartis: Consultancy, Honoraria, Speakers Bureau; Samyang: Research Funding; Roche: Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; BMS: Honoraria, Speakers Bureau; Abclon: Consultancy; Takeda: Honoraria, Speakers Bureau; Beigene: Consultancy; Boryung: Research Funding; Kirin Pharm: Honoraria, Speakers Bureau; GI cell: Consultancy; GC cell: Consultancy. Kim:Samsung Bioepis: Consultancy; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Uncompensated relationship; Regeneron: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Uncompensated relationship; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; IMBDx, Inc.: Honoraria, Speakers Bureau; Boryung: Consultancy, Other: Uncompensated relationship; BeiGene: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Honoraria, Other: Uncompensated relationship, Research Funding; F. Hoffmann-La Roche Ltd: Consultancy; MedImmune: Consultancy, Honoraria, Other: Uncompensated relationship; Yuhan: Consultancy; Amgen: Honoraria. Chung:Progeneer Incorporation: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal